

 |
 |
|
|---|---|---|
| I & C Technical Guides | ||
More technical details can be found in our publication in the Journal of Microscopy (2009, Volume 235, Pt 2, 128-137)
Choosing the technology
Microbes that express a fluorescent protein or are stained with fluorescent dyes can be used to image living biofilms with fluorescence microscopy. This can be either standard microscopy, single photon laser scanning confocal microscopy (SP-LSCM) or multiphoton laser scanning confocal microscopy (MP-LSCM).
There are several advantages of MP-LSCM over SP-LSCM some of which are
The biofilm is a highly scattering sample so for the above reasons we chose to use multiphoton laser microscopy.
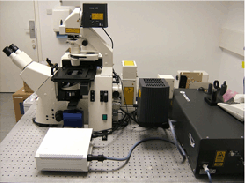
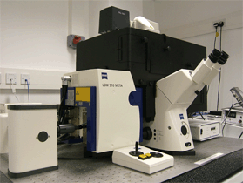
Imaging was carried out on a Zeiss LSM 510 NLO META LSCM using a bespoke configuration with three non-descanned detectors on the reflective port (NDD, left hand image) coupled to a Coherent Chameleon Ultra laser (centre image). This was fitted onto a Zeiss Axiovert 200 microscope in a fully enclosed, blacked out, bespoke temperature controlled chamber (Solent Scientific, right hand image). Images were acquired using various objectives including a Plan Apochromat 63x/1.4 oil DIC objective, C-Apochromat 40x/1.1W Corr UV-VIS-IR objective , C-Achroplan NIR 40x/0.8 objective and a W Plan Apochromat 20x/1.0 DIC VIS-IR objective
 |
 |
 |
|---|
Hardware modifications
When fully assembled the injection ports and outlet ports on the sides of the flow cell prevented the flow cell from resting flat on the microscope stage. One solution to this problem was to raise the flow cell using a bespoke stage insert. However, by raising the flow cell, the objective could no longer reach the coverslip and a threaded riser was also required. Together these modifications (shown below) allowed the flow cell to be placed on the microscope stage and full X, Y plane mobility retained and the plane of focus could be reached throughout the flow cell depth.
 |
 |
 |
 |
 |
Flow cell with side ports |
Bespoke stage insert |
Objective lenses and |
Flow cell on microscope stage |
|
Variable Parameters that affect the image
These are discussed in context of multiphoton laser scanning confocal microscopy but some will also be applicable to single photon-LSCM imaging.
Laser power
This is a
compromise between the desire to use high laser power to visualize even faintly fluorescent material and low laser power to avoid inducing unintentional damage/alterations to the sample. Increased laser power causes fluorescence bleaching, death of the biofilm, ablation and even localized water expulsion or “explosions” in extreme cases.
Gain
This is
a compromise between increased signal and increased noise. In the cases where high gain is necessary, multiple scans can be performed and by using frame averaging so the noise is filtered out.
Fluorescence
The wavelength of the signal should be considered, since both the excitation and emission of longer wavelengths (red) can be obtained to greater depth than the shorter (green) wavelengths. An example is shown in our publication. This would be of particular concern in very thick biofilms (also see below).
Fluorescent proteins
All the normal variables associated with the systems should be carefully considered, such as intensity, half life, maturation time and whether the coding sequence is suitable for the organism.
Fluorescent dyes
The concentration of the dyes used is important. In some instances we noticed increased fluorescence after attempting to ‘bleach’ an area which can be attributed to excitation of excess dye.
Keep in mind the biology
What is the parameter being reported and how might biofilm growth (physiology, heterogeneity) affect the signal or the interpretation of the signal. For example, there is an ongoing discussion about the cells that stain “dead” with BACLight Live/Dead and whether they are indeed always non-viable.
Biomass
Biomass
limits the signal output. Biofilm age and the growth medium may affect the quality of the image. Growth rates will differ with medium composition, and thus age is not an “absolute” variable.
Imaging pitfalls - Things you may see that are artifacts of imaging.
A lack of a signal in an image is readily interpreted as absence of biofilm material but care has to be taken that it is not a case of lack of fluorescence intensity.This is particularly important when interpreting results using two colors of considerably different wavelength. Using the BACLight Live/Dead stain one can interpret the image incorrectly: all cells will take up SYTO 9 (green) but only the cells with altered membrane integrity will take up PI (“dead”). Thus the interpretation is based on the fact that the red signal overpowers the green signal. As mentioned above, red signal can be detected to greater depths, and thus it is possible that all the visible cells are “dead” (red) but that the live cells that are present (green) in the same plane are just not visible. Similar problems could theoretically arise with a mixed biofilm of GFP and tdTomato cells, etc.
In some instances individual bacteria proved to be considerably brighter than others. If imaging is optimised on the bright bacteria the dim bacteria become invisible and this leads to misinterpreted data so to is important to ensure all cells can be visualised. Autofluorescence can be discounted or controlled by using an unstained specimen.
| Imaging and Cytometry Laboratory Technology Facility, Department of Biology University of York, PO Box 373 York, YO10 5YW, UK |
Last modified on 3 August 2009 by Jo Marrison |